Blog
This is your complete guide to healthcare MVP prototyping, which involves turning your idea into a functional, test-ready medical product.
Introduction – The New Era of Health Innovation
Healthcare is no longer just the domain of large corporations and research labs. Over the past decade, advances in rapid prototyping, digital manufacturing, and integrated electronics have made it possible for startups, clinicians, and even solo inventors to bring new medical solutions to life faster than ever.
In this landscape, one concept has become a game-changer: the Minimum Viable Product (MVP). An MVP is the first, functional version of your medical or health-tech product—stripped down to its core features—designed to validate your idea in the real world. It’s the bridge between a great concept and a market-ready device.
At Warning Machines, we help innovators take their vision from care—that first spark of wanting to improve health outcomes—to cure—a tested, tangible product in the hands of users. This article will guide you through the process of creating your health MVP story and how to make it resonate with investors, partners, and end-users.
Why Healthcare MVP Prototyping Works for Faster Medical Innovation
Developing healthcare products—whether they’re wearable devices, diagnostic tools, or assistive technologies—comes with unique challenges: strict regulations, high production costs, and the need for rigorous testing.
The MVP approach addresses these challenges in three ways:
Speed to Market
By focusing only on essential features, you can create a functional prototype in weeks, not years. This allows you to test viability before investing heavily.Risk Reduction
Building an MVP lets you identify design flaws, compliance issues, and user-experience problems early, when they’re still affordable to fix.Investor Confidence
A working MVP speaks louder than a pitch deck. It shows that your idea isn’t just theoretical—you can build it, test it, and scale it.
Step 1 – Define the Problem, Not the Product
Every strong health MVP story begins with a clear, validated problem. It’s tempting to start with your invention’s features, but in healthcare, the why matters more than the what.
Ask yourself:
What is the exact pain point you’re solving?
Who feels this pain the most?
How is it currently being addressed, and why isn’t that good enough?
Example: Instead of saying, “We’re building a new insulin delivery system”, you might frame it as:
“Patients with Type 1 diabetes often face inconsistent insulin absorption due to current device limitations. Our MVP focuses on a delivery method that maintains stable glucose levels with less manual intervention.”
Step 2 – Map Your Core Functionality
Once you’ve defined the problem, strip your solution down to its essentials. Your MVP should do one thing exceptionally well—anything more adds time, cost, and complexity.
For a healthcare MVP, this often means:
One core mechanical function (e.g., precise dosing, accurate measurement)
One clear user interface (e.g., single-button operation, simple mobile app)
Basic data capture or reporting if needed for validation
At Warning Machines, we help clients through Design for Manufacturing (DFM) reviews to ensure even the earliest prototypes can be scaled later.
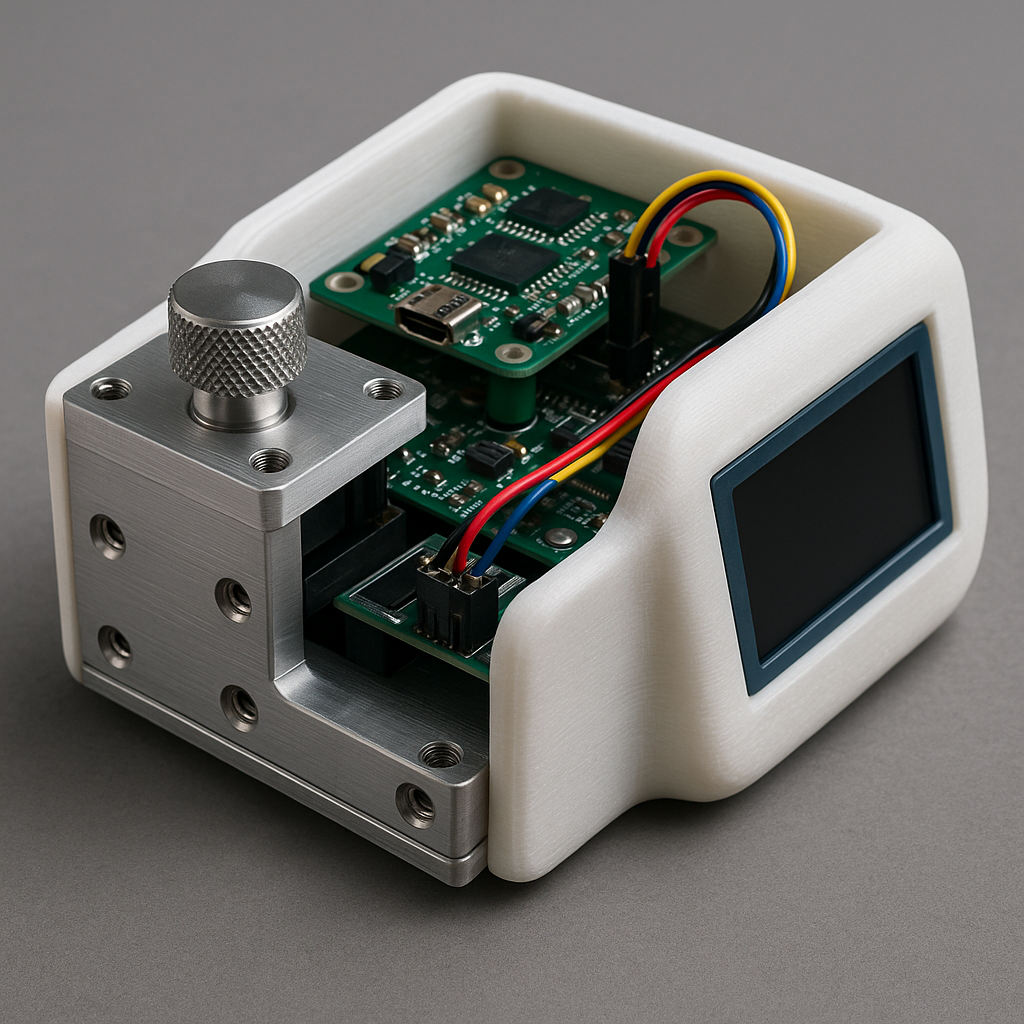
Step 3 – Choose the Right Prototyping Methods
The tools and materials you choose will directly affect the cost, accuracy, and speed of your MVP. Common approaches in health MVP development include:
CNC Machining for precision metal or plastic components like surgical tools or device housings.
3D Printing (FDM, SLA, SLS) for fast, cost-effective iteration of complex geometries.
PCB Design & SMT Assembly for wearable sensors, diagnostic electronics, or connected devices.
Integrated Testing Fixtures to simulate real-world usage and gather performance data.
Warning Machines offers all these services under one roof, meaning your project won’t suffer delays from outsourcing or supplier mismatches.
Step 4 – Validate with Real Users
Healthcare innovation doesn’t succeed in isolation. The earlier you can put your MVP in front of real users—clinicians, technicians, patients—the better.
User validation can include:
Usability testing with simulated patients
Clinical environment trials for workflow fit
Feedback sessions with medical professionals
The goal here isn’t just to prove your MVP works—it’s to uncover what you didn’t know you didn’t know.
Step 5 – Document Your Story for Compliance and Investment
In healthcare, documentation isn’t just nice to have—it’s mandatory. From ISO 13485 standards to FDA or CE marking pathways, the story of your MVP’s development should include:
Design history (sketches, CAD, design iterations)
Test results (performance, safety, reliability)
User feedback reports
Manufacturing process notes
This documentation also becomes part of your investor pitch, showing you’ve thought beyond the prototype toward full-scale production.
Crafting the “Care to Cure” Narrative
Your MVP isn’t just a physical object—it’s a story. And in health innovation, a compelling story can be as important as the technology itself.
A strong narrative includes:
The Human Element – Start with the patient, the healthcare worker, or the underserved population you’re helping.
The Breakthrough Moment – Explain what led you to create this solution. Was it a personal experience, a clinical observation, or a technological insight?
The Journey – Share the steps you’ve taken, the challenges you’ve faced, and how your MVP overcomes them.
The Vision – Describe what “cure” looks like in your context. Is it eliminating a symptom, reducing hospital stays, or enabling earlier detection?
Case Example – From Clinic Idea to Working Device in 30 Days
Imagine a physiotherapist who notices patients struggling with adherence to home exercise programs after surgery. She sketches an idea for a wearable motion tracker that gives real-time feedback.
With Warning Machines:
Week 1: We run a DFM review, create CAD models, and define essential electronics.
Week 2: 3D print the housing, CNC machine wearable clips, and produce the first PCB.
Week 3: Integrate firmware and run initial tests with mock users.
Week 4: Deliver a functional MVP ready for clinical pilot testing.
What’s Next? Move from MVP to Regulated Product
Once your MVP is validated, the next steps involve scaling manufacturing, refining the design for reliability, and navigating regulatory pathways (e.g., IEC 60601/62304, ISO 13485). This is where a disciplined approach pays off—each iteration should be documented, tested, and aligned with the end goal of patient safety and efficacy.
Ready to Build Your Health MVP?
Whether you’re prototyping a diagnostic device, a wearable sensor, or a digital therapeutic, we can help you turn your healthcare idea into a validated MVP—fast.
Let’s bring your health innovation from care to cure.
 Warning
Warning